Fungitell®
 O kit Fungitell® é um teste de diagnóstico altamente sensível e rápido que detecta
O kit Fungitell® é um teste de diagnóstico altamente sensível e rápido que detecta
(1 → 3) -ß-D-glucano no soro em apenas uma hora.
A capacidade do teste Fungitell® de detectar níveis de picograma de (1 → 3) -ß-D-glucano no soro, auxilia aos médicos na identificação da doença de fungos invasivos (IFD: Invasive Fungal Disease) no início do processo da doença.
Generalidades
O teste Fungitell® mede os níveis de (1 → 3) -ß-D-glucano no soro. O reagente de detecção é uma cascata biológica baseada numa modificação do Limulus Amebocyte Lysate (LAL), um extrato das células sanguíneas do caranguejo Horseshoe Crab da América do Norte.
O reagente Fungitell é modificado para eliminar o Fator C e, assim, ignorando o fator B ativado, apenas reage com os (1 → 3) -ß-D-glucanos, através do Fator G. Isso torna o reagente altamente específico para (1 → 3) -ß-D-glucanos e não reage com outros polissacarídeos, incluindo beta-glucanos com diferentes ligações glicosídicas.
A detecção de (1 → 3) -ß-D-glucanos não está sujeita às habituais interferências. Não é suprimida pela terapia anti-fúngica, nem o teste é reativo com outros polissacarídeos.
(1 → 3) -ß-D-glucano em fungos patogênicos
A maioria dos fungos patogênicos * tem (1 → 3) -ß-D-glucano nas paredes celulares. Durante a infecção quantidades mínimas de (1 → 3) -ß-D-glucanos são liberadas na circulação.
A detecção de níveis elevados de (1 → 3) -ß-D-glucano é uma ajuda para o diagnóstico presuntivo de doença fúngica invasiva em pacientes com risco.
Resultados rápidos
O ensaio Fungitell® é realizado inteiramente dentro de um poço de microplaca sem passos de lavagem.
O ensaio fornece resultados dentro de 2 horas.
Cascata enzimática
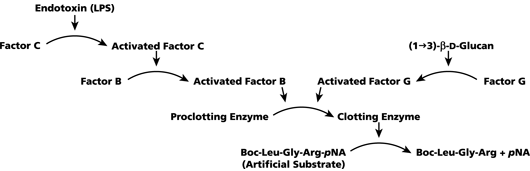
Quando o (1 → 3) -ß-D-glucano está presente numa amostra, ativa o Factor G, um zimogênio da serina da protease . O Factor G ativado converte a enzima de proclotagem inativa para a enzima ativa de coagulação, que por sua vez cliva o pNA do péptido cromogênico do substrato ,Boc-Leu-Gly-Arg-pNA, criando um cromóforo que absorve a 405 nm.
O reagente é usado no ensaio cinético Fungitell, para detectar a taxa de aumento da densidade óptica em uma amostra. Esta taxa é interpretada contra uma curva padrão para produzir estimativas da concentração de (1 → 3) -ß-D-glucano na amostra.
Semelhante aos imunoensaios enzimáticos, o ensaio Fungitell é realizado em microplacas e lido em um leitor de incubação. Ao contrário dos imunoensaios enzimáticos, o ensaio Fungitell não exige nenhuma etapa de lavagem. Todos os reagentes adicionados permanecem no poço. O teste Fungitell pode ser executado em aproximadamente uma hora.
Em maio de 2004, a FDA liberou o kit Fungitell para venda, para auxiliar no diagnóstico de infecções fúngicas. O teste Fungitell também foi marcado CE para a venda na Europa em março de 2008.
Significado Clínico
Há uma incidência crescente de infecções por fungos patôgenicos primários e oportunistas, especialmente em pacientes imunocomprometidos. As doenças fúngicas invasivas, como infecções oportunistas, são comuns entre os pacientes com malignidade hematológica e AIDS e representam um número crescente de infecções nosocomiais, particularmente entre os receptores de transplante de órgãos e outros pacientes que recebem tratamentos imunossupressores.
Os patógenos fúngicos humanos primários comuns são Candida spp e Aspergillus spp , Patógenos fúngicos oportunistas, incluindo Fusarium spp, Trichosporon spp, Saccharomyces cerevisiae, Acremonium, Coccidioides immitis, Histoplasma capsulatum, Sporothrix schenckii e Pneumocystis carinii.
O (1 → 3) -ß-D-glucano produzido por estes organismos pode ser detectado pelo ensaio Fungitell.
O soro humano normal contém baixos níveis de (1 → 3) -ß-D-glucano, tipicamente 10-40 pg / mL, presumivelmente a partir de leveduras comensais presentes no canal alimentar e no trato gastrointestinal. A maioria dos fungos patogênicos tem (1 → 3) -ß-D-glucano nas paredes celulares e pequenas quantidades são espalhadas na circulação durante o ciclo de vida. Assim, o (1 → 3) -ß-D-glucano aparece no soro em casos de infecção por fungos invasivos (IFI).
Monitorar a glucanemia sérica para evidenciar níveis elevados e crescentes fornece um marcador substituto conveniente para IFI. Níveis acima de 80 pg / mL, em pacientes em risco, são considerados positivos.
O Teste Fungitell é indicado para o diagnóstico presuntivo de infecção por fungos. Ele deve ser usado em conjunto com outros procedimentos de diagnóstico. O ensaio Fungitell não detecta certas espécies de fungos como o gênero Cryptococcus, que produz níveis muito baixos de (1 → 3) -ß-D-glucano. Este ensaio também não detecta os Zygomycetes, como Absidia, Mucor e Rhizopus, que não são conhecidos por produzir (1 → 3) -ß-D-glucano.
Pacientes com alto risco para Infecção por Fungos (IFD)
Múltiplos estudos mostraram que o glucano se elevou bem antes dos sinais e sintomas clínicos convencionais. O diagnóstico precoce de infecção por fungos está associado a um melhor resultado clínico e é um valor para os médicos clínicos.
- Em contraste, o diagnóstico e terapia tardios da doença fúngica invasiva estão associados ao aumento da mortalidade. Portanto, há uma utilidade significativa na aplicação do teste Fungitell® em pacientes com risco.
- Os pacientes imunossuprimidos estão em alto risco de desenvolvimento de doença fúngica invasiva, que muitas vezes é difícil de diagnosticar. As populações de pacientes afetadas incluem:
- Pacientes com câncer submetidos a quimioterapia
- Pacientes com células estaminais e transplante de órgãos
- Pacientes queimados
- Pacientes com HIV
- Pacientes na UCI (Unidade de Cuidados Intensivos)
Amostras de pacientes : amostragem recomendada
As amostras de soro devem ser recolhidas em tubos de vácuo estéreis, deixadas coagular e depois centrifugadas para separar o coágulo do soro, isto é, durante aproximadamente 1800 rpm durante 10 minutos numa centrífuga clínica. Remover a amostra do soro sob condições aceitáveis e conservar alíquotas em vials de armazenamento. O teste para um paciente requer 0,5 mL de soro.
O soro é o único tipo de amostra que está atualmente aprovado para uso com o ensaio Fungitell. O soro que é hemolizado, lipêmico ou visualmente icterico ou turvo não é adequado para uso com o ensaio Fungitell.
Como uma infecção por fungos é um processo dinâmico se deve repetir o teste, normalmente 2-3 vezes por semana, melhora a sensibilidade.
Os cursos de tempo dos níveis séricos de BG (Beta glucanos) ao longo de uma infecção demonstraram apresentar um nível ascendente que cai em resposta a uma terapia eficaz.
Valores de Referência
- Negativo: Menos de 60 pg / mL
- Indeterminado: 60-79 pg / mL
- Positivo: > / = 80 pg / mL
O ensaio Fungitell é indicado para o diagnóstico presuntivo de infecção por fungos. Ele deve ser usado em conjunto com outros procedimentos de diagnóstico.
O ensaio Fungitell não detecta certas espécies de fungos como o gênero Cryptococcus, que produz níveis muito baixos de (1 → 3) -ß-D-glucano. Este ensaio também não detecta os Zygomycetes, como Absidia, Mucor e Rhizopus, que não são conhecidos por produzir (1 → 3) -ß-D-glucano.
Diagnóstico clínico – Informações de referência
Resumos de referência
Os seguintes artigos estão disponíveis mediante solicitação. Para solicitar uma reimpressão de texto completo do artigo, preencha o formulário de solicitação de informações. Certifique-se de indicar qual artigo você está solicitando. Devemos ter um endereço de correspondência para enviar o artigo. As reimpressões dos artigos só estão disponíveis na forma impressa.
Alexander BD.
Transplant Infectious Diseases Services, Clinical Mycology Laboratory, Duke University Medical Center, Durham, NC 27710, USA. alexa011@mc.duke.eduAbstract: The dramatic increase in nosocomial invasive mycoses over the past two decades has led to increased interest in the area of antifungal development. Unfortunately, the infusion of new diagnostic technology into the clinical mycology laboratory has lagged behind. Although newer, automated, continuous-monitoring blood culture systems are as sensitive as the older, manual “gold standard” system, the recovery of fungi from blood, as well as other clinical specimens, remains an insensitive marker for invasive fungal infection. Antigen assays for the rapid diagnosis of invasive fungal infections are in development, and galactomannan and glucan are two such promising antigens. Glucan may be present in the blood of patients with infection secondary to a wide variety of fungal pathogens, including Candida, Aspergillus, Fusarium, Saccharomyces, trichosporon and Acremonium species. Early data suggest galactomannan may be present in the blood in detectable levels very early in the course of invasive aspergillosis. The galactomannan assay currently undergoing evaluations may actually be positive prior to the clinical suspicion for infection and may be useful in monitoring therapeutic response as well; however, the etiology of false-positive results following cytotoxic chemotherapy still has to be elucidated. PCR assays are also being developed in the research laboratory, however, the PCR assays will require a significant amount of adaptation and validation before they are ready for clinical care. Well-planned studies to evaluate the performance characteristics as well as appropriate clinical and cost-effective application of these new tests are needed.
T Miyazaki, S Kohno, K Mitsutake, S Maesaki, K Tanaka, N Ishikawa, and K Hara
Second Department of Internal Medicine, Nagasaki University School of Medicine, Japan.
(1→3)-ß-D-Glucan is one of the major structural components of fungi, and it seems that it can be detected by the fractionated (1→3)-ß-D-glucan-sensitive component from a Limulus lysate, factor G. We evaluated the concentration of (1→3)-ß-D-glucan by using factor G and other fungal antigens in 24 patients with clinical evidence of mycosis and 36 healthy subjects. The mean concentration of (1→3)-ß-D-glucan in the plasma of the healthy subjects was found to be 2.7 +/- 1.9 pg/mL (range, < 6.9 pg/mL), and it was found to be substantially higher in all 11 patients with candidemia (mean, 2,207.4 pg/mL; range, 325.4 to 8,449.0 pg/mL). Eight of those 11 patients with candidemia (73%) were positive for the Cand-Tec heat-labile candida antigen and only 3 patients (27%) were positive for mannan antigen. Three patients with invasive pulmonary aspergillosis were positive for galactomannan and had, in addition, high concentrations of (1→3)-ß-D-glucan (mean, 323.3 pg/mL; range, 27.0 to 894.0 pg/mL). All 10 patients with cryptococcosis (including 2 patients with probable cryptococcosis) were positive for cryptococcal antigen by the Eiken latex test; however, (1→3)-ß-D-glucan levels were not elevated in these patients (mean, 7.0 pg/mL; range, < 16.5 pg/mL). Our results indicated that (1→3)-ß-D-glucan levels are elevated in patients with candidiasis and aspergillosis but not in those with cryptococcosis.
Jones BL,McLintock LA.
Department of Medical Microbiology, North Glasgow Hospitals University NHS trust, Royal Infirmary, University of Glasgow, Glasgow, UK. b.l.jones@clinmed.gla.ac.uk
Purpose of Review
The early treatment of invasive fungal infection is critical but is hampered by the non-specific nature of clinical and radiological signs and the insensitivity of current laboratory diagnostic methods. If mortality due to invasive fungal infection is to be reduced, new, preemptive therapeutic strategies, targeting those patients at highest risk, are required and these will depend on the development of rapid, sensitive diagnostic methods. Such methods have become available in the form of high-resolution computed tomography scanning and serological and molecular techniques and in this review the authors describe recent studies assessing the utility of these methods and consider their role in management strategies.
Recent Findings: Sensitive assays for the detection of fungal DNA and antigens such as galactomannan and glucan have been prospectively evaluated in the clinical setting and enable identification of patients at an earlier stage of infection. However, the sensitivity and specificity of the assays vary considerably in different studies, depending on several factors including patient selection and clinical application of the test, and issues regarding the release and circulation of galactomannan and fungal DNA remain to be clarified.
Summary: Rapid serological and molecular diagnostic methods facilitate the early diagnosis of invasive fungal infection and would appear to be most useful when used prospectively to screen high-risk patients. However, in order to determine the optimal approach to treatment it is essential that these tests are incorporated into management strategies and their impact on incidence of invasive fungal infection and clinical outcome evaluated in further clinical trials.
Odabasi Z, Mattiuzzi G, Estey E, Kantarjian H, Saeki F, Ridge RJ, Ketchum PA, Finkelman MA, Rex JH, Ostrosky-Zeichner L.
Laboratory of Medical Mycology, University of Texas-Houston Medical School, Houston, TX, USA.
The Glucatell (1→3)-ß-D-glucan (BG) detection assay (Associates of Cape Cod) was studied as a diagnostic adjunct for invasive fungal infections (IFIs). On the basis of findings from a preliminary study of 30 candidemic subjects and 30 healthy adults, a serum BG level of >or=60 pg/mL was chosen as the cutoff. Testing was performed with serial serum samples obtained from 283 subjects with acute myeloid leukemia or myelodysplastic syndrome who were receiving antifungal prophylaxis. At least 1 serum sample was positive for BG at a median of 10 days before the clinical diagnosis in 100% of subjects with a proven or probable IFI. IFIs included candidiasis, fusariosis, trichosporonosis, and aspergillosis. Absence of a positive BG finding had a 100% negative predictive value, and the specificity of the test was 90% for a single positive test result and >or=96% for >or=2 sequential positive results. The Glucatell serum BG detection assay is highly sensitive and specific as a diagnostic adjunct for IFI.
Obayashi, T., Yoshida, M., Mori, T., Goto, H. Yasuoka, A., Iwasaki, H., Teshima, H., Kohno, S., Horichi, A., Ito, A., Yamaguchi, H., Shimada, K., and Kawai, T. (1995)
Summary
(1→3)-ß-D-glucan is a characteristic fungal cell-wall constituent. To assess the clinical usefulness of this glucan in screening for invasive fungal infection or fungal febrile episodes, we measured the plasma concentration at the time of routine blood culture in 202 febrile episodes by means of factor G , a horseshoe-crab coagulation enzyme that is extremely sensitive to this polysaccharide.
With a plasma cut-off value of 20 pg/mL, 37 of 41 episodes of definite fungal infections (confirmed at necropsy or by microbiology) had positive results (sensitivity 90%). All of 59 episodes of non-fungal infections, tumour fever, or collagen diseases had concentrations below the cut-off value (specificity 100%). Of 102 episodes of fever of unknown origin, 26 had plasma glucan concentrations of more than 20 pg/mL. With those 102 cases taken as non-fungal infections, the positive predictive value of the test was estimated as 59% (37/63), the negative predictive value as 97% (135/139), and the efficiency as 85% 9172/202). The positive predictive value should improve if there were a sensitive gold standard that could discriminate fungal from non-fungal infections. Causative fungi included candida, aspergillus, cryptococcus, and trichosporon.
Determination of plasma (1→3)-ß-D-glucan with factor G is a highly sensitive and specific test for invasive deep mycosis and fungal febrile episodes, and will substantially benefit immunocompromised patients.
Yuasa K., and Goto H. (1997)
Division of Hematology, Department of Medicine and Department of Clinical Pathology, Jichi Medical School, Minamikawachi-machi, Tochigi-ken, Japan, 329-04
Abstract: To elucidate the role of (1→3)-ß-D-glucan in pulmonary aspergilloma, serum concentrations of (1→3)-ß-D-glucan were measured repeatedly for as long as 10 months in eight patients. In four patients with inactive disease, concentrations of (1→3)-ß-D-glucan were in the normal range.The concentrations of (1→3)-ß-D-glucan increased in two patients, although the disease was inactive. This increase might show the earliest stage of the invasive process of the disease. In two other patients with active disease, (1→3)-ß-D-glucan increased. Other parameters, such as galactomannan, immunodiffusion and a radio-allergosorbent test, as well as inflammatory markers such as C-reactive protein and the leukocyte count, did not show any consistent tendency in regard to the activity of the disease. Thus, a (1→3)-ß-D-glucan assay may add valuable data for evaluating the disease activity and understanding the disease process of pulmonary aspergilloma.
Yoshida M, Obayashi T, Iwama A, Ito M, Tsunoda S, Suzuki T, Muroi K, Ohta M, Sakamoto S, Miura Y. (1997)
Department of Medicine, Jichi Medical School, Tochigi-ken, Japan.
Abstract: (1→3)-ß-D-glucan, a characteristic fungal molecule, is known to increase in blood in invasive candidiasis, aspergillosis and cryptococcosis. This report shows that the plasma glucan concentration was also elevated in four patients infected with Fusarium, trichosporon beigelii, Saccharomyces cerevisiae and Acremonium. In three of the patients, the elevation preceded positive blood cultures by 5-17 days, and in one of them it even preceded the onset of fever by 6 days. In a fourth patient, the glucan level decreased with clinical improvement. Plasma (1→3)-ß-D-glucan determination appears to be useful also for diagnosis and follow-up of these unusual deep mycoses.
Pazos C, Ponton J, Del Palacio A.
Unidad de Micologia, Departamento de Microbiologia, Hospital Universitario 12 de Octubre, Avenida de Cordoba s/n, 28041 Madrid, Spain. pazos.c@terra.es
Abstract: Two noninvasive diagnostic tests, (1→3)-ß-D-glucan (BG) (Glucatell) and galactomannan (GM) (Platelia Aspergillus), were used retrospectively in a twice-weekly screening for the diagnosis of invasive aspergillosis (IA) in 40 treatment episodes (one hospital visit per patient) in 40 neutropenic adult patients at high risk for IA. Five proven IA cases, three probable IA cases, and three possible IA cases were diagnosed. Diagnostic levels of both BG and GM were detected in 100% of patients with proven IA cases and in 66% of patients with probable IA cases. The kinetics of both markers in patients with IA were similar. The sensitivity, specificity, and positive and negative predictive values for GM and BG were identical, namely, 87.5, 89.6, 70, and 96.3%, respectively. False-positive reactions occurred at a rate of 10.3% in both tests, but the patients showing false-positive results were different in each test. Both tests anticipated the clinical diagnosis, computed tomography abnormalities, and the initiation of antifungal therapy in most patients, but BG tended to become positive earlier than GM. A combination of the two tests improved the specificity (to 100%) and positive predictive value (to 100%) of each individual test without affecting the sensitivity and negative predictive values. In conclusion, BG and GM detection are useful tests for the diagnosis of IA in high-risk hematological patients, but a combination of the two tests was very useful to identify false-positive reactions by each test.
Links de Recursos
Dr. Fungus
The Aspergillus Website
NIH – Medline Plus
PubMed
Esses links são fornecidos apenas para informação. Associates of Cape Cod, Inc. Não é responsável pelo conteúdo dos links de recursos externos. Somente os operadores das páginas vinculadas são responsáveis pelo seu conteúdo.


